Requirements for Patentability of Selection Invention
(Superordinate Concept and Subordinate Concept)
I. Trial for Invalidation of Patent Registration No. 33727
Patent Court’s Decision No. 2004HEO6521 published on Nov. 3, 2005
Supreme Court’s Decision No. 2005HU3338 published on Sep. 6, 2007
- dismissed
1. Present patented invention
(1) Claim 11
A compound of the formula (I) or (I’).
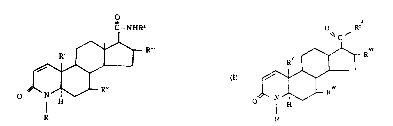
(Since a substituent is not related to a point at issue, the description of the substituent is omitted.)
(2) Claim 16
A compound of claim 11which is 17β (N-t-butylcarbamoyl)-4-aza-androst-1-ene-3-one.
2. Patent Court’s decision (2004HEO6521)
(1) Requirements for patentability of a selection invention
① Where a constitutional requisite is described as a superordinate concept in a prior or publicly known invention, a selection invention has only a subordinate concept included in the superordinate concept as all or part of the constitutional requisite. The present invention described in claim 16 is the selection invention relating to the compound of 17β (N-t-butylcarbamoyl)-4-aza-androst-1-ene-3-one, which corresponds to the subordinate concept of ‘4-aza-17β-substituted-5α-androstan-3-one’ of the cited invention, which is a substance of the superordinate concept.
② A selection invention can be patentable where, first, a prior invention does not concretely disclose the subordinate concept constituting the selection invention, and second, all subordinate concepts included in the selection invention have the effects qualitatively different from those of the prior invention, or even if there are no qualitative differences, there are qualitatively remarkable differences. It is enough if the detailed explanation of the selection invention clearly describes that the selection invention has the aforementioned effects compared with the prior invention. It is not required that comparative experimental data to specifically confirm the remarkability of the effects shall be described. If the effects of the selection invention are doubtful, the applicant may concretely assert and prove the effects by a method of submitting the concrete comparative experimental data after the filing of the application of the selection invention.
③ In general, the effects of the invention described in the specification of the invention are enough by disclosing the results made according to the constitution of the invention as the cause and effect. Especially, in a chemical substance invention, in many cases, the substance itself corresponds to the constitution of the invention in light of the properties, and the actual manufacturing of the substance corresponds to the effects of the invention. If an inventor proves only the point that (s)he can actually manufacture the substance having the constitution, such as, examples, element analysis values, melting points, refractive indexes and so on, the effects of the invention are considered as being described as the descriptive requirements of the specification of the invention.
However, the selection invention cannot be patentable because it essentially corresponds to a duplicate invention of the prior invention. Nevertheless, to promote the use and improvement of a foundation invention and to promote the development of industries and the enhancement of public interests, a patent is exceptionally given to the (selection) invention formed of the subordinate concept having the excellent effects which are not specifically recognized by the prior invention as the superordinate concept. Therefore, it is not proper to apply the general criteria in determining whether the description in the specification is legal or not. Only if the specification clearly discloses whether the selection invention clearly has the special and outstanding effects which are not recognized by the prior invention, the selection invention is considered as satisfying the descriptive requirements of the specification of the selection invention.
④ Establishment of a selection invention, and determination of inventive step thereof
The criteria for remarkability of the effects to approve the inventive steps of the selection invention is very strict, compared with that of a general invention. Thus, it is required to prove that the example(s) of the selection invention is excellent, compared with all example(s) of the prior invention. In this regard, it is not fair to require that all data satisfying the aforementioned conditions shall be included in the specification at the time of filing the application of the selection invention. When the inventive steps of the selection invention are at issue, the inventor(s) can prove that his/her selection invention has the excellent effects by the submission of comparative data at a later time. Only in this meaning, the data of the selection invention can be submitted later. It does not mean that the descriptive requirements for the effects of the invention are satisfied just by the specification which includes the abstract expression indicating that the inventor(s) has obscurely perceived differences in the effects.
⑤ In a medical substance invention, the invention is generally formed by the steps of substance→pharmacological activity→medical use. The step of substance→pharmacological activity is proved by in vitro tests or various in vivo tests including animal tests, organ tests, cell tests or the like. The step of pharmacological activity→medical use generally uses animal tests. In vitro tests usually indicate the results by using numerical values. In vivo tests also statistically indicates whether any reaction exists or indicates it by using relative numerical values compared with a prior medical substance, so that the vivo tests are able to quantitatively indicate the results. Therefore, where the inventor, who describes at least that the effects of the selection invention ‘is excellent compared with the prior invention’, completes the selection invention by clearly recognizing the quantitatively remarkable effects at the time of filing the application, instead of describing such a point without objective recognition of the effects, the inventor must have the materials based for his/her recognition and decision of the effects. Thus, the inventor can describe the effects in the specification by using the quantitative numerical values.
⑥ The Supreme Court’s precedent
According to the Supreme Court’s precedents, in medicine, the objective description of the acting effects (medical effects) of a medicine is the descriptive requirement. Thus, if the detailed explanation of the invention does not describe the medical effects of the invention described in the claims, the specification is considered as having descriptive defects. In a numerical value defining invention, the specification shall clearly describe the critical effects based on the numerical value definition. In a medical use invention, unless there is the special reason that the pharmacological mechanism indicating the pharmacological effects is clearly proved before the filing of the application, the specification shall provide examples with pharmacological data indicating a specific substance has the pharmacological effects, or a concrete description capable of replacing the examples.
In the numerical value defining invention or medical use invention, it is different from the selection invention as a substance invention in terms of the category. However, at any case, these inventions have a fundamental common point that these inventions can be patented exceptionally even through these inventions are within the scope of the conventional and publicly known art. In the numerical value defining invention or the medical use invention, the precedents require that the different and remarkable effects of the invention shall b
|